India Celebrates Its First Locally Developed CAR T-Cell Therapy For Treatment Of Cancer
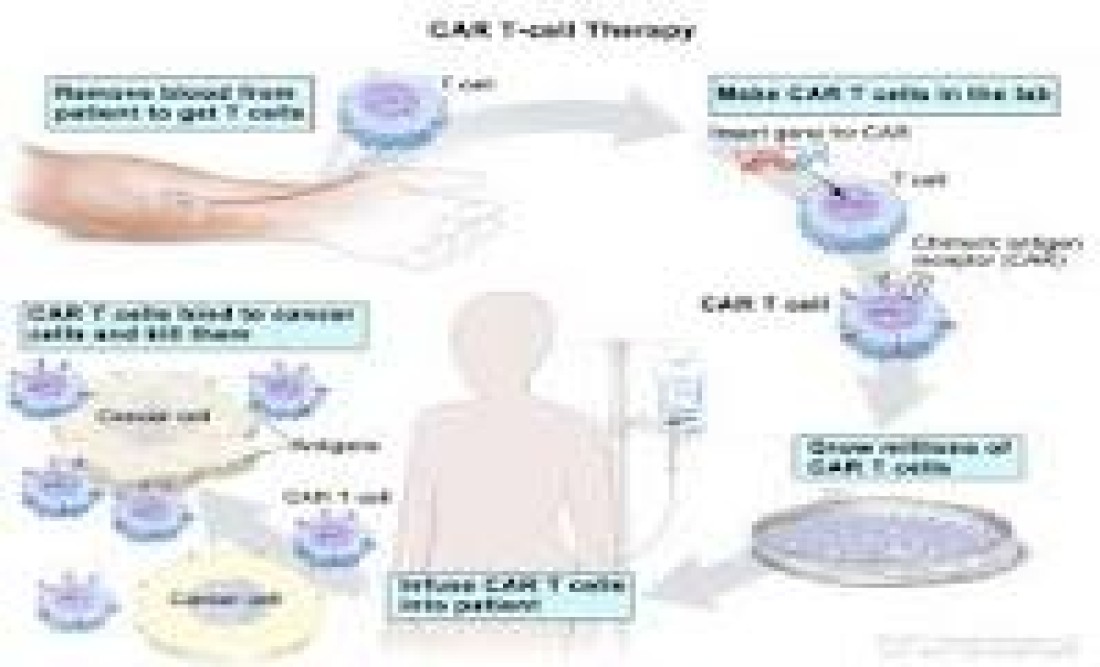
New Delhi 25 April 2024 (VNI) In a significant development for India's healthcare sector, President Droupadi Murmu unveiled the nation's initial indigenous chimeric antigen receptor (CAR) T-cell therapy on April 4th. The therapy, known as actalycabtagene autoleucel (actaly-cel, marketed as NexCAR19), also called chimeric antigen receptor T-cell therepy, signifies a notable advancement in the treatment of cancer, especially in the realm of blood cancers.
CAR-T therapies, which entail modifying a patient's T cells (a kind of immune system cell) in the laboratory to target and destroy cancer cells, have quickly emerged as a promising treatment choice for specific cancer types. Historically, these therapies have been prohibitively expensive, with expenses in the United States frequently exceeding $400,000 per dose. Nonetheless, India's locally developed CAR-T therapy is poised to transform this scenario by offering a more affordable alternative, with a single NexCAR19 treatment costing between US$30,000 and $40,000, about one-tenth of the cost of comparable global commercial products.
The development of actaly-cel is the outcome of collaborative efforts among scientists at the Indian Institute of Technology, Bombay, and Tata Memorial Hospital, in association with industry partner ImmunoACT. In October 2023, India's Central Drugs Standard Control Organization authorized actaly-cel for use in treating relapsed or refractory B-cell lymphomas and leukemia, making it the first CAR T-cell therapy approved for use in the country.
Speaking at the unveiling event held at IIT Bombay, President Murmu praised the indigenous development of actaly-cel as an exemplary instance of the Make in India initiative. She highlighted the therapy's potential to considerably reduce the cost of treatment, thereby improving access to cutting-edge cancer care for patients across the country.
The introduction of India's first domestically developed CAR T-cell therapy marks a significant milestone in the country's healthcare journey. It not only demonstrates India's growing capabilities in the field of medical research and innovation but also underscores the nation's commitment to providing affordable and accessible healthcare solutions to its citizens. As actaly-cel becomes more widely available, it is expected to bring new hope to cancer patients and their families, marking a new chapter in India's fight against cancer.
No comments found. Be a first comment here!









